NexCAR19 therapy was developed for B-cell blood cancers by ImmunoACT (a company incubated under IIT Bombay, and Tata Memorial Hospital), supported by DBT and BIRAC.
- NexCAR19, India’s 1st indigenous CAR T-Cell therapy, was granted market authorization by the Central Drugs Standard Control Organisation (CDSCO) in 2023.
About CAR (Chimeric Antigen Receptor) T-Cell Therapy
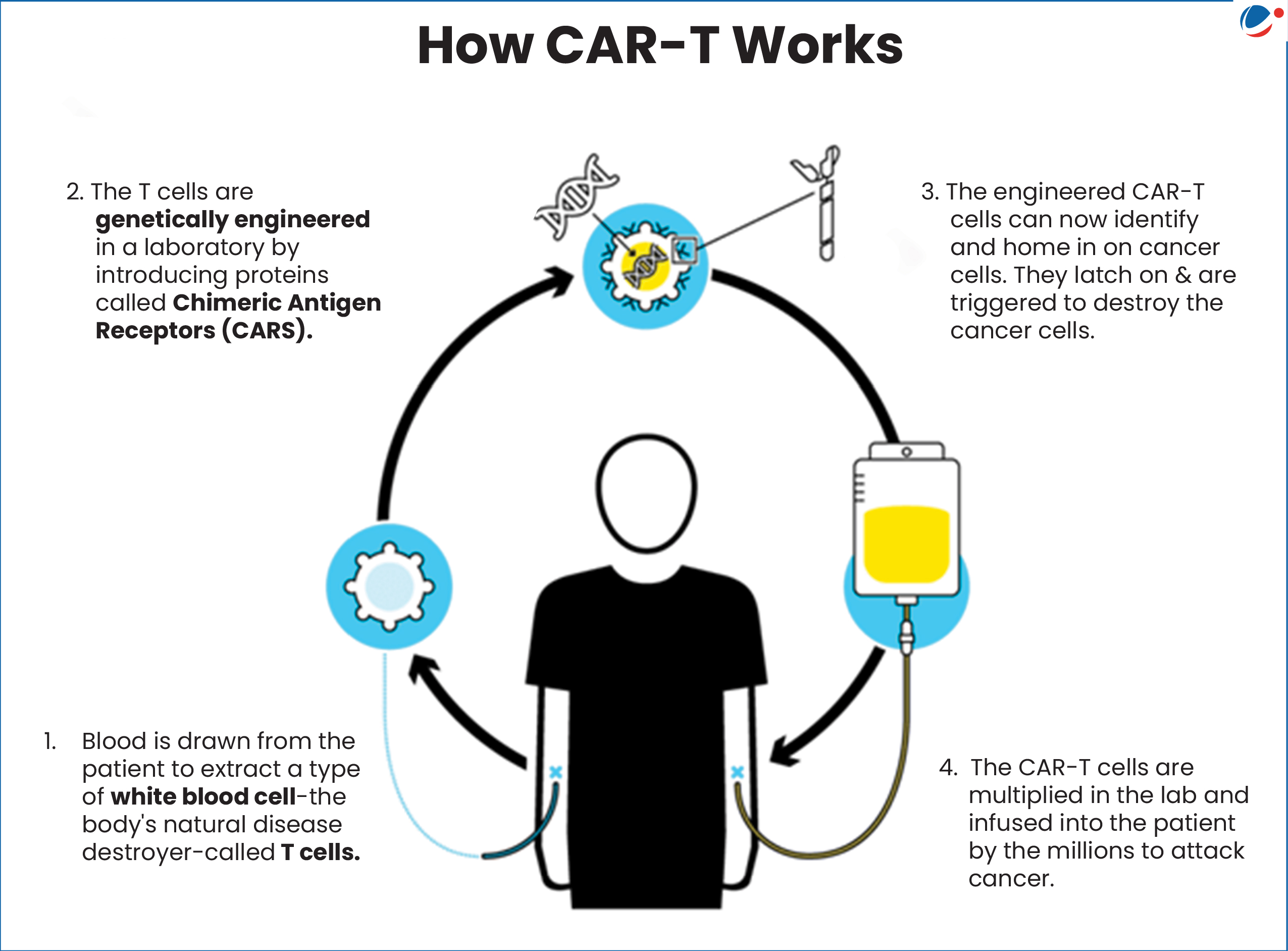
- It modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- T-cells are special cells (types of white blood cells) whose primary function is cytotoxic, meaning killing other cells.
- This treatment is designed for specific types of blood cancer and is given to patients whose cancer has either relapsed or not responded to first-line treatment.
Benefits of CAR T-Cell therapy
- Short treatment duration: Unlike aggressive chemotherapy or stem cell transplants, it allows for a faster recovery.
- Sustained Benefits: CAR T-cells persist in the body, offering long-term protection against cancer relapse.
- Accessibility: Nexcar19 cost is lower compared to imported CAR-T therapies.
Challenges: Therapy for one cancer won't work for another type of cancer, can have negative effects on the nervous system, Cytokine Release Syndrome (Over activation of immune cells), risk of infection, etc.




