Ocean acidification refers to a reduction in the pH of the ocean over an extended period of time, caused primarily by uptake of carbon dioxide (CO2) from the atmosphere.
- Ocean acidification has been called the "evil twin of global warming" and "the other CO2 problem".
Ocean Acidification Process
- Carbon dioxide (CO2) from the atmosphere dissolves in the ocean, creating aqueous carbon dioxide (CO2 (aq)).
- This dissolved CO2 reacts with water (H2O) to form carbonic acid (H2CO3).
- Carbonic acid then breaks down into bicarbonate (HCO3-) and hydrogen ions (H+).
- The increase in hydrogen ions makes the ocean more acidic.
Causes of ocean acidification
- Ocean absorbs around 25% of annual anthropogenic CO2 Emission.
- Natural CO2 emission like Submarine Volcanic Activity, Ocean Circulation, Ocean Sediment Breakdown etc.
Impact of Ocean Acidification
- Acidic waters lead to dissolution of calcium carbonate structures (Affecting organisms like corals, shellfish, and plankton) and Ecosystem alterations affecting fisheries and coastal protection.
- Ocean acidification harms phytoplankton, thus reducing ocean’s productivity.
- Ocean acidification can indirectly affect cloud formation by impacting the production of dimethyl sulfide (DMS) by phytoplankton.
- DMS is a sulfur compound that acts as a cloud condensation nucleus, meaning it helps form cloud droplets.
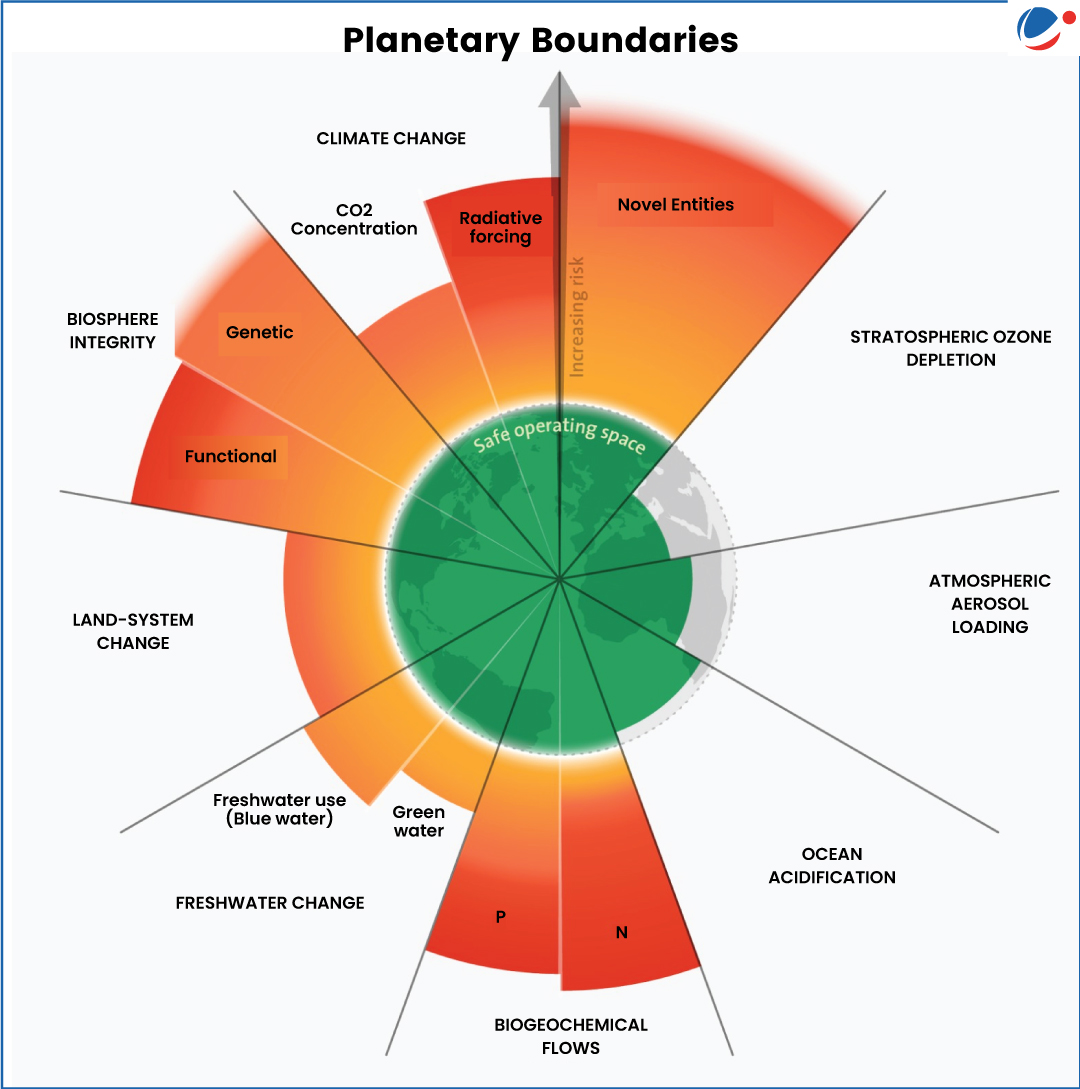
Planetary Boundaries
|






