Why in the News?
Susumu Kitagawa, Richard Robson and Omar M. Yaghi are awarded the Nobel Prize in Chemistry 2025 for the development of metal–organic frameworks.
More on the News
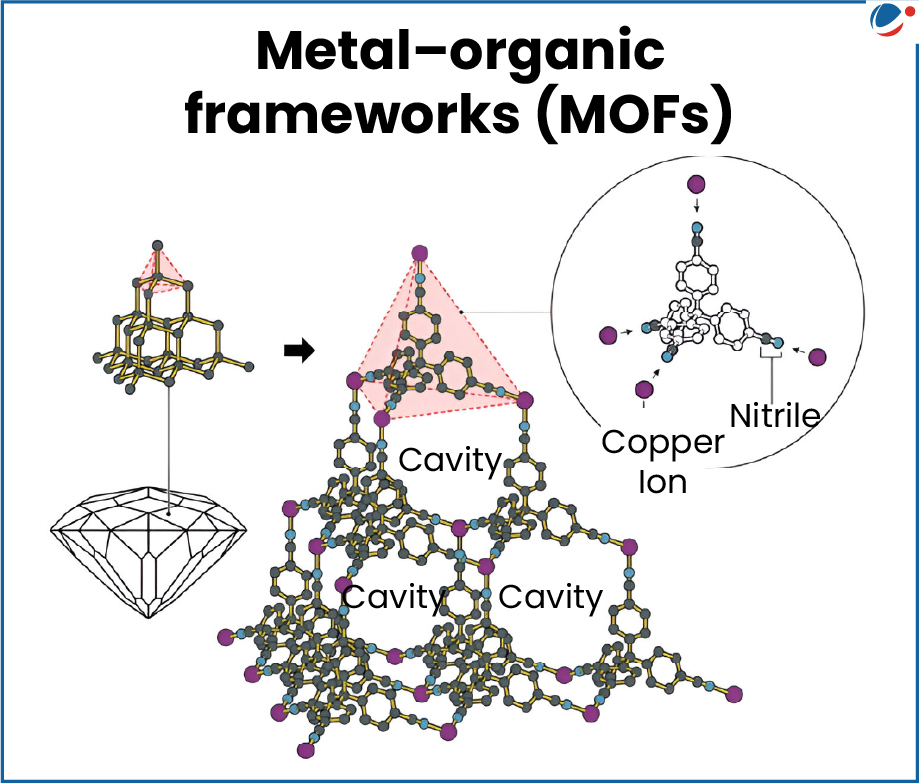
- The laureates have created molecular building blocks called metal organic frameworks (MOFs) having large spaces through which gases and other chemicals can flow.
What is Metal–Organic Framework (MOF)?
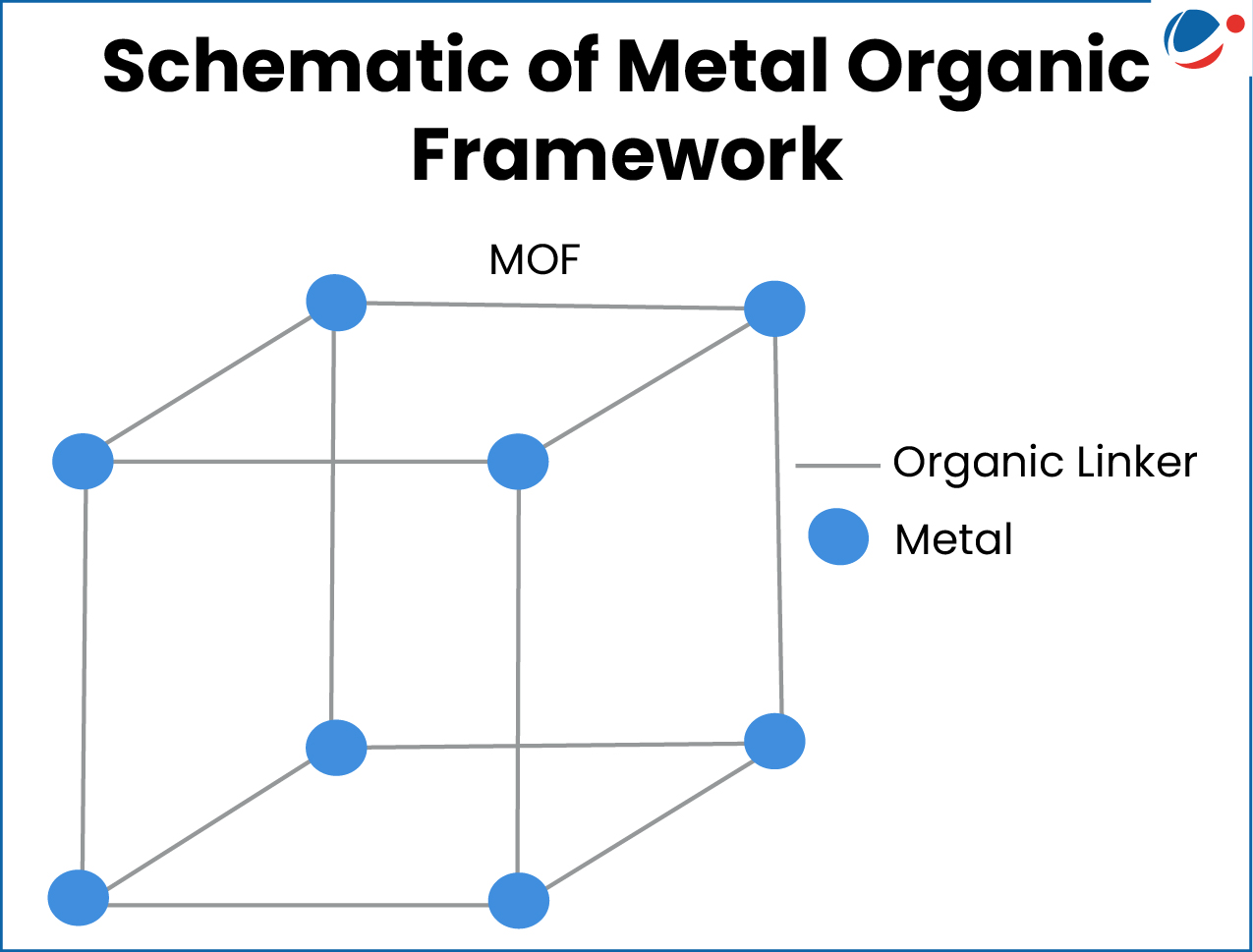
MOFs are special materials made up of two parts:
- Metal ions (such as copper, zinc etc.) – this act like the "joints" or "connecting points"
- Organic molecules (carbon based molecules like coal, glucose etc.) – these act like the "links" or "bridges" that connect the metal parts together.
- When metal ions and organic molecules join through chemical bonds, they form a network structure.
- The network structure can be in one, two, or three dimensions — just like lines, sheets, or 3D frameworks with a lot of empty spaces or cavities between them.
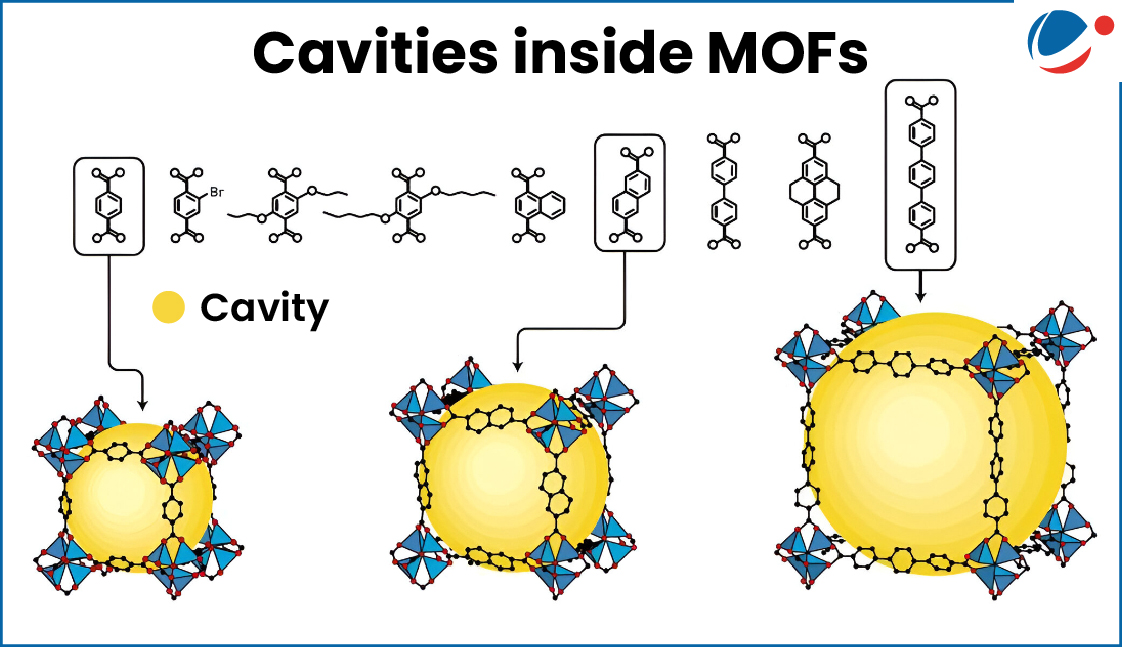
- The cavities inside the network make the material highly porous- full of tiny holes inside.
- For example (see infographic): Copper metal (shown as dot) combines with the organic molecule (shown as honeycomb like structure) to produce a 3D chain like framework with cavities.
- Another example(see infographic)- The metals are like bricks in a wall and organic molecules are like pillars which connect one brick to another.
- When bricks and pillars are arranged periodically they form spacious molecular rooms.
- The size and shape of these molecular rooms can be changed by changing the bricks (metals) and pillars (organic molecules).
Origin of the Idea of MOF
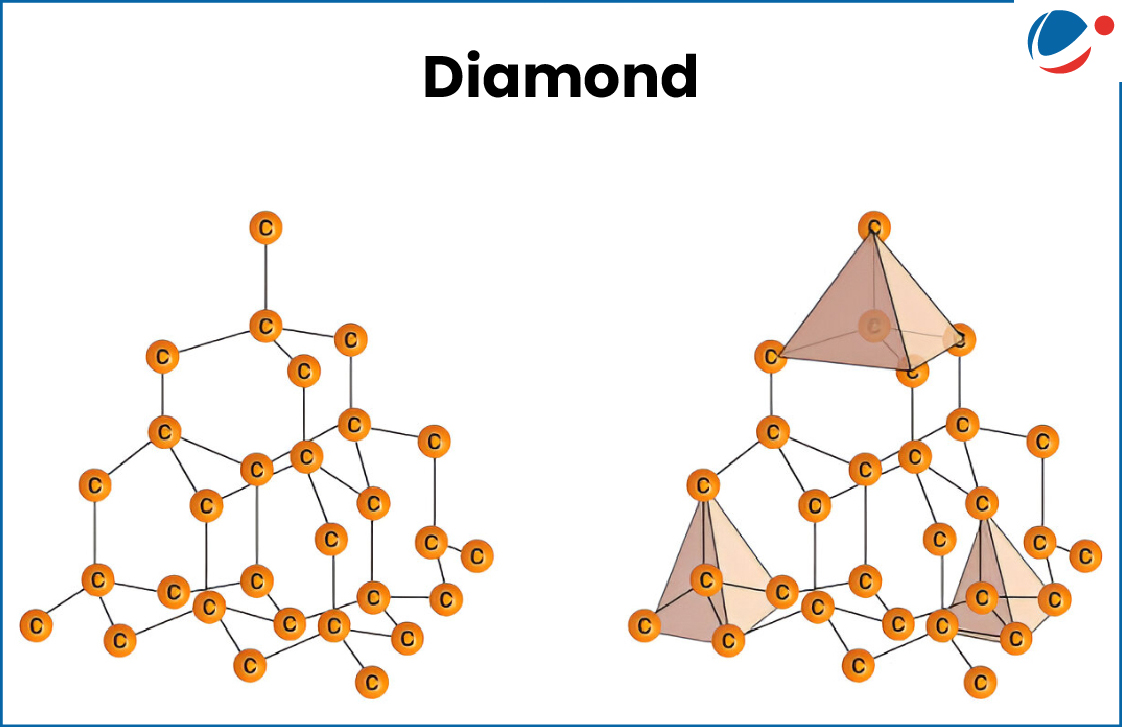
- The idea to create MOFs came from studying the structure of diamond - in which each carbon atom connects to four others, forming a small pyramid-like pattern.
- Scientists used a similar concept but replaced carbon with metal atoms and organic molecules to create these new porous materials.
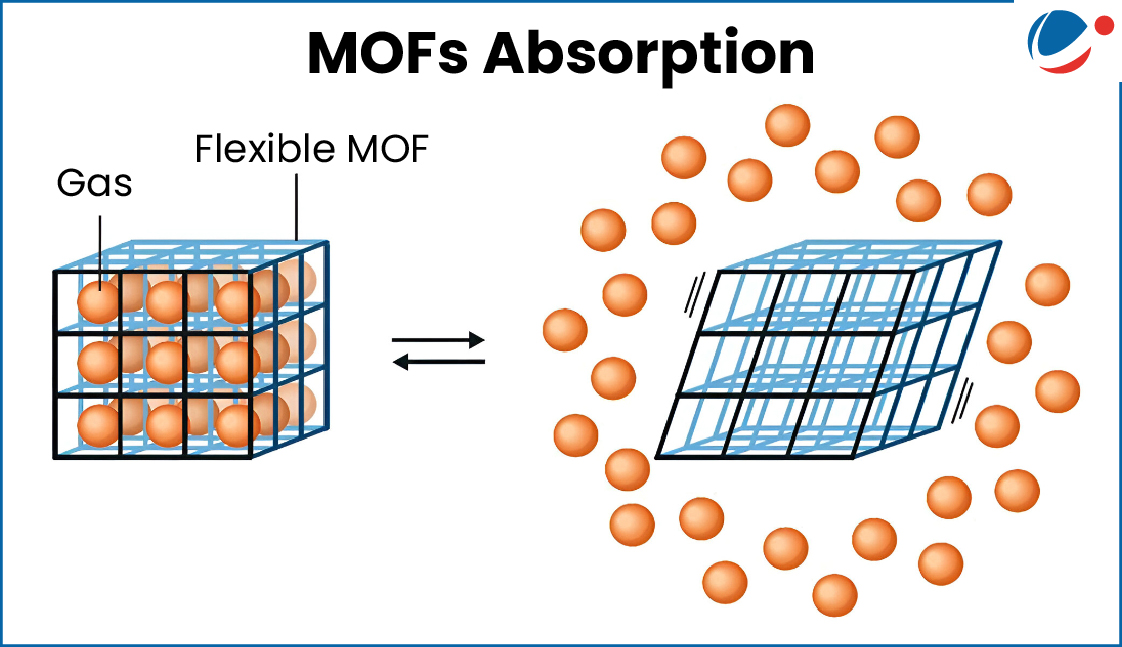
Key Features
- The pores or cavities inside MOFs can be made bigger or smaller by changing the metals or the organic molecules used.
- This means MOFs can be customized to absorb or trap specific kinds of materials like gases, water, or chemicals.
- One gram of MOF can have an internal surface area roughly equal to two football fields - showing how spacious it is inside.
- Because of their large cavities and surface area, MOFs are more absorbent than many other porous materials (like zeolites).
- They act like molecular storehouses, storing gases, liquids, or other materials inside their pores.
Key Applications
Their ability to trap, store, or release molecules makes them useful for:
- Water collection from dry air
- Water in desert regions is scarce. The concentration of water in the air is low which makes several existing methods of water collection from air ineffective.
- MOFs have large cavities with good absorption capacity. Membranes made of MOF material can absorb large quantity of water.
- After the absorption, liquid water can be efficiently extracted by slightly heating the MOF membrane. Thus, MOFs can solve the problem of water shortage in desert areas.
- Food Processing
- Fruits and vegetables release ethylene gas (it is a hormone) which causes ripening. After the harvesting, the release of ethylene is significantly increased which causes their spoilage.
- MOF based packaging materials have strong absorption capacity which stores the ethylene gas into its cavity.
- This absorption reduces the concentration of ethylene and increases the shelf life of fruits and vegetables.
Other Similar Applications
- Pollution control: Extracting pollutants, such as separating PFAS, antibiotics etc. from polluted water.
- Rare-Earth Elements: Experimentally mining rare-earth elements from wastewater.
- Gas storage: Storing gases such as hydrogen, carbon dioxide, methane etc.




